
Myths and mis-information regarding Condensation and Moulds
There are Company websites proclaiming they are ‘experts in condensation and mould issues’. They state things like: ‘Get the professionals in’, ‘The condensation experts’. But should you believe them?
How do you know if they are just a website that states ‘pseudo facts’ that are nothing more than just myths? Even Local Councils and Housing Associations get taken in.
Look at some of their 'facts' and compare with actual proven science:
Opening windows
‘Open the window and let all the steam out’ – It helps very slightly, however doesn’t do much at all to reduce the condensation around the home. As mentioned in a previous blog, dry air comprises atoms of nitrogen (78%), oxygen (21%) and trace gases (1%) with space in between them.
Humidity is the addition of H2O molecules diffused in the air. The molecules float in the spaces between the other atoms. In the image on the right it indicates 'saturated air' at about 100%RH.
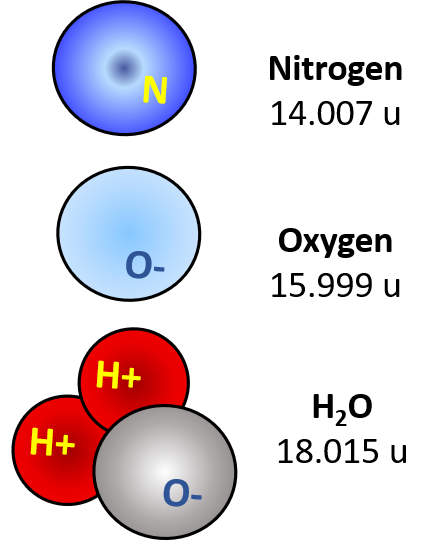

Humid air is dry air with water molecules (vapour) in the spaces. H2O can be solid as ice, liquid as water or gas as vapour. The molecules are exactly the same with a ratio of 2 hydrogen atoms to 1 oxygen atom (H2O). They remain as singular molecules whilst in gas / vapour format.
Water can vaporise at temperatures well below boiling point (100°C). The evidence is water evaporates from the oceans and seas etc.
Leave a glass of water and eventually the water will evaporate. (If you want to find out more it will be in Construction Science Explained – www.buildabooks.co.uk out soon).
The atoms of a water molecule remain the same whether it is water, ice or vapour. As vapour they can float in the spaces between the other gases in the air. That process is termed humidity.
A common myth-statement: ‘Steam is water vapour’ . Not true. Steam is water. It is water vapour that has condensed in the air therefore, it is actually condensation and visible. Gases including water vapour are not visible.
Molecules are more than one atom. They can be the same type or different types termed compounds. Atoms have weight termed 'Atomic weight' that is a comparison with a carbon atom. They have a mass that can be attracted by a greater mass. The greater the mass the stronger the attraction. (Similar to weight).
Weight as a verticle force perpendicular to the Earth's surface is commonly referred to as 'gravity'. However, substances with large numbers of atoms attract substances with lessor numbers of atoms. The force is perpendicular to the centre of the larger substance. It can be in any direction.

The Science Museum in London had a demonstration of the force in action between two steel balls suspended on steel wires down a stair well.
The two steel balls attracted each other and the force could be measured by comparing the distance between the cable at the ceiling and between the suspended balls]. The force was at right angles to Earth's gravity.
The planet Earth is made up of an enormous number of atoms. The combined attraction (pulling force) we call ‘gravity’. It’s a bit like a very large magnet will attract a smaller item such as a nail. The nail if magnetised will attract a smaller pin, and so it goes on down the sizes.
Gravity however is a strong attractive force that can go through most things and still attract. It can go through the tallest buildings and still have an effect on an aircraft flying at 35,000 feet in the air. So logically it also has an attractive force on every atom be it in a solid (the aircraft), a liquid (rain comes downwards) and the gases.
If it didn’t have any effect on the gases they would all have gone off into outer space. Irrefutable evidence: the higher the altitude the less molecules. We state that the air is ‘thinner’. What it actually means there are less molecules, less nitrogen, less oxygen etc. and a lot more space.
So what has all this proved?
Air can be dry – no water vapour in it. Very rare though, most air has some water vapour in it even in the hottest driest deserts.
Atoms have a mass. (Mainly the Protons and Neutrons. Electrons are so small they are considered as having no mass). The more mass in the same volume means there are more particles for gravity to act on. We term that ‘weight’. If you could take say a gold bar that has a weight on Earth of 6kg then send it to the Moon it would then only weigh about 1kg. The gold bar hasn’t changed, it still has exactly the same number of atoms in it. The only difference is the attraction force ‘gravity’. The Moon is that much smaller, so less atoms to to do the attraction.



If air is heated, the atoms and molecules will become more energised. They will move more quickly and collide with each other. The collisions tend to end up as the molecules bouncing off and going in a different direction to their next collision. All the time that is happening gravity is trying to pull them down to the lowest point and slows them down. The result is less collisions = less heat generated = the air cools down.
If a cubic metre of air is considered, there will be a given number of air molecules in it at a specific temperature. If the molecules are given more energy (electromagnetic energy) they become more energised and travel faster and further. There will be fewer molecules in the cubic metre as the temperature rises. Fewer molecules means the pull of gravity has less effect. The cooler air will push the warmer less dense air upwards. Warm air rises and that is the reason why.
Evidence: the attractive force of gravity can be compared to magnetic attractive force. The closer together metal particles are the easier the magnet can attract them (ignore friction).
Warm air is less dense than cooler air as there are fewer molecules for gravity to act on. Warm air therefore is displaced by cooler air that has more molecules in the given volume. The end result is warmer air will be pushed upwards by the cooler air trying to get as low as possible.
That was considering dry air only; Nitrogen, oxygen and trace gases argon and carbon dioxide.
Now add water vapour.
Water vapour (H2O) has mass so will be attracted by gravity. The molecules fill the spaces between the other atoms by diffusion. Eventually the molecules fill the spaces and the air becomes saturated at 100%RH.
Nitrogen and Oxygen are both diatomic atoms. That means they naturally float around bonded in pairs. (N2 & O2). The atomic mass therefore is doubled making Nitrogen about 28 amu and Oxygen about 32 amu. When compared with the H2O molecule they are both 'heavier'.
In theory, that suggests that humid air rises as stated on various Websites. However, there are other factors to consider: Temperature and pressure. The atoms and molecules are not stationary floating in space. They are bouncing off each other at phenominal speeds. When the H2O are highly charged they will bounce around and be pushed upwards by the cooler air by displacement.
As the energy reduces by transfer to cooler surfaces, plus gravitational pull, the molecules will lose momentum and the atomic attraction will pull them closer together. The consequence is the vapour becomes liquid = condensation. Although the molecules have not changed their atomic structure they have slowed down, therefore less collisons and less thermal output. H2O molecules naturally try to bond with other H2O molecules in preference to other atoms. In liquid form they will also try to bond with other atoms and molecules that are polar such as silica.

The gravitational pull on Nitrogen is greater than hydrogen. Hydrogen has 1 Proton and no Neutrons. Whereas Nitrogen has 7 Protons, 7 Neutrons and 7 Electrons.
The number of Protons and Neutrons govern the atomic attraction indicated as Atomic Mass.
An atom of Nitrogen has an atomic mass of about 14 amu. In contrast, Oxygen is about 16 amu and Hydrogen about 1 amu.
All three atoms are diatomic, meaning they are naturally bonded in pairs.
Therefore:
- Hydrogen is about 2 amu
- Oxygen is about 32 amu
- Nitrogen is about 28 amu
H2O is about 18 amu
It is said that humid air ‘rises’ but it is actually it is being pushed upwards by the cooler more dense air. (Nothing can go upwards without energy being used, not even atoms).
So dry air is more dense than humid air at the same temperature. The warmer the air the fewer number of molecules therefore they get pushed upwards (rise). *[Avogadro's theory relates to the number of particles in a given volume (Mole). Particles are the smallest matter, they make up the atoms. The theory suggest that a mole of any substance at the same temperature and pressure will contain the same number of particles].
The air in the shower will be saturated with water vapour. At its maximum temperature the humid air will be pushed upwards by the cooler air. If an extractor in the ceiling or high up the wall is close to the shower outlet it will pull the humid air out of the room.
The hot water will be flowing over the person and surfaces of the materials around the shower (walls, shower curtains, panels etc.). Those surfaces will be cooler than the hot water but as there is so much water any condensation will blend in with the water.
The surfaces will warm up via heat transfer from the hot water. They will also cool the water down in the process. (example; water at 40°C and surface at 20°C will become about 30°C)
The air around the shower area will be cooler still and condensing the vapour into steam. The visible condensation in the air, steam will further condense on cooler non-porous surfaces to form globules of water. The molecules of the surface material attract the water until there are too many water molecules to support and the globule is pulled downward. Commonly known as ‘streaming’.
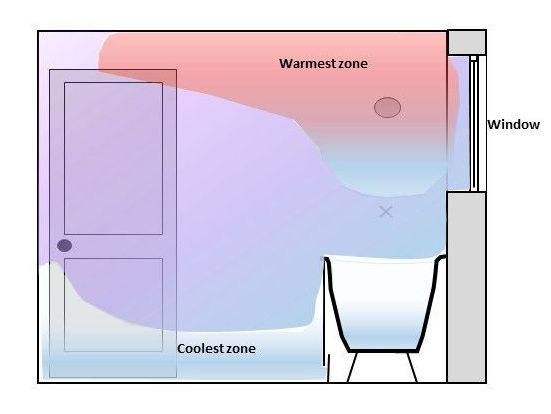
Naturally the humid air in the bathroom will be warmer than the ambient air temperature and therefore be pushed upwards as it is less dense.
The cooler air has more density and displaces the less dense air.
Having a clear gap about 10mm below the door and a working extractor fan, the humid air will be pulled out of the room.
It is beneficial to have the fan operating BEFORE the shower is used. There will be an air flow formed that will reduce the humid air from filling the room.

The bathroom / en-suite door if closed is keeping much of the humid air in that room. The extractor is pulling the humid air out at a design speed (we will come back to that in a moment). Air, humid or dry cannot be pulled out of a room unless other air is going in to replace it. (otherwise you would end up with a vacuum).
'Mind the gap!' The gap under the door
If the gap under the door is at least 10mm (3/8”) it will let enough air in to the bathroom / en-suite to replace the humid air being pulled out.
For those who are interested: If the door is 762mm wide (2’-6”) then the gap area will be 7,620mm². A pipe 100mm diameter will have a cross sectional area of 7,854mm² which is slightly more meaning there will be a greater negative pressure but not enough to worry about.
The gap is normally at the lowest level in the room where the air is at its coolest and most dense. It cannot escape under the door as there is a negative pressure (suction) as the extractor is pulling the air out of the room.
Why do I need to know all that?
Simply having an efficient extractor running before the shower goes on (or running a hot bath) will start the negative pressure so any humid air will be going out of the extractor outlet as opposed to building up in the room.
Opening a window – Hmmm.
Based on the science it isn’t going to do much. The main objective is to pull the humid part of the air out of the building and opening a window will not do that.
Having an extractor going with the window open isn’t much good either. It is possible that the RH during the hotter days or wetter days is greater than 60%RH. Opening a window will hardly help to reduce much water vapour by diffusion.
The window is unlikely to be at floor level so all that cooler humid air will still be there. Open the door and it can then roll out into the rest of your home.
[On the day of writing it is a very hot and dry day in June 2025. The outside relative humidity level is 56%RH with a temperature of 27°C. There is also a strong breeze. The RH in the house is also 56%RH as the H2O molecules diffuse into the house air].

Bottom line: An efficient extractor operating before the humidity rises in the room. Door shut and the drier warm air from the rest of your home is used to replace the very humid air being extracted.
Extractor continues to operate for about 30mins after the shower has stopped. (Door still closed or just very slightly open). That will remove the very humid air that is formed by water evaporation. By wiping up as much as possible any water or condensation left on the hard surfaces around the shower it will reduce further humidity.
An experiment: Use a highly absorbant cloth to wipe up as much condensation and water from all the hard surfaces including the bath or shower tray. Wring the cloth out into a container to catch all the water. That would have all had to evaporate into the air. Leaving the extractor on for 30 mins will help to remove the rest of the water you haven’t mopped up. (Shower curtains tend to hold a lot of water on the surfaces).
1cc of water is equal to about 1 gram at 20°C. To enable easier comparison between moisture content and relative humidity a psychrometric chart has been developed. A graph-like chart enables comparison between grams of moisture per kg of dry air. For example: at 20°C 40%RH will contain nearly 6 grams of water per kg. Increase the moisture by about 3g to about 9g of moisture and the RH increases to 60%RH.
Compare the room temperature at a more realistic 25°C:
40%RH will be about 8g of moisture. 60%RH will be 12g and at 80%RH about 16g.
[Air mass: about 1.2kg per cubic metre]
The comparisons indicate as the air temperature increases more humidity can be held in dry air. In contrast, as the temperature falls, less vapour can be held = condensation. It is therefore important that as much of the hottest water vapour is removed by the extractor before it cools down.
Extractor efficiency
The manufacturers provide figures showing how many cubic metres of air an extractor fan is capable of pulling down a tube per second, per minute, or per hour. Some extractor fans have an adjustable amperage meaning the amount of energy used to turn the blades of the fan. More revolutions normally equals more of an air flow.
The humidity will fill the whole volume of the room and all volumes of containers in it such as drawers, cupboards, wardrobes and that includes the waste tubes in the shower tray, bath, basin and inside the pan and water closet of the WC.
Air naturally contains pollutants including mould spores as mentioned in a previous blog.
Mould requires moisture to enable it to absorb food. No moisture = no absorbed food = no growth.
Mould can grow down inside the plug hole tube and into the water trap. A slime builds up and the mould feeds off of that. The tell-tale sign that mould and slime are growing is commonly the smell.
Bleach will kill the mould and to a point dislodge the slime from the pipe. It ONLY applies to the mould spores the bleach comes into contact with.
Ideally pour the equivalent of a tea cup full down the plug hole and leave it overnight. Then put the plug in and fill the basin with water (hot or cold it doesn't really matter - however hot tends to work better. When the basin is full, pull the plug out and let the water flush the waste pipe through.
It is surprising the amount of slime and hair that will be flushed out. A bath trap can produce about a cup full yet still allow water to go through albeit slowly.
Water is not pure H2O. If it were then you wouldn’t want to drink it as it would taste horrid. Tap water contains minerals that are dissolved in water. Calcium for example naturally occurs in water. It is an essential mineral for our bones and all other creatures that have bones or shells. Chalk is basically Calcium (Calcium Carbonate) formed from the bones of billions of creatures over thousands or millions of years.
Another mineral naturally occurring in water is Magnesium.
Magnesium calcite is derived from chalk (the skeletons of those billions of sea creatures). When rain water (which is slightly acidic) goes into the ground it dissolves minute parts of the chalk. A bit like putting sugar in a hot cup of tea. The sugar dissolves and it all becomes liquid.
Potassium is also a naturally occurring mineral in the ground and dissolves in water.
How do the liquefied minerals help mould to grow?
Compare minerals in water with sugar in a cup of tea. Leave a cup of tea and eventually the water content will evaporate into the air (that‘s if no-one drinks it first). The sugar in the tea cannot evaporate and the molecules join together to form crystals. The crystals are very hard and tend to be brittle. They will bond to most hard surfaces producing a coating of sugar crystals.
Now compare that to the water from the tap. Water that contains a large quantity of minerals in solution (dissolved) is termed ‘hard water’. The minerals are in solution, i.e. in liquid form. The molecules are attracted to other molecules causing bonds.
Calcium is a naturally occurring element, a metal by definition. It can exist in water as solution, i.e. liquid, or as a solid commonly termed limescale or calcite. The higher the temperature of the water or the material the water is touching boosts the bond. The result is the calcium solidifies and coats the material. The most common form is calcite (calcium carbonate). It is a form of limestone giving the term limescale. Basically it is a stone-like coating that slowly attracts more calcium out of solution.
In something like a washing machine or dishwasher where the cold water is heated via a metal heating element the formation of calcite in hard water will occur. (That will be the subject of a future blog – Is it cost effective to stop calcite forming in hard water areas?). The minerals can also bond to other surfaces even as cold water.

Calcite will also form when the water molecules evaporate. If you live in a hard water area your kettle heating element probably ‘scales up’ – has a crust of calcite coating it.
There is also probably calcite around the outlet of the taps and plughole material. If there has been a leaking or dripping tap there will be a deposit of calcite where the water has been.
Calcite will bond to most surfaces. Plastics, metals, ceramics etc. Mould of various strains are in the water supply naturally. The spores of others are naturally in the air.
Mould generally feeds on carbon atoms. Calcite is a carbonate (CaCO3) - contains carbon. Therefore moulds have moisture and food and will replicate. In the photo there are at least 3 different strains of mould on the plastic pipe in the loo water box.

What else is in water? Without putting you off drinking water, sufficient to say, there are lots of things. Some are in solution / dissolved, and others remaining as microscopic solids in suspension / floating around.
Will bleach kill them all?
Short answer is no. The water supply companies use various methods to kill off most of the bacteria. However, some will still survive and is acceptable in very small amounts. Algae for example. To prove it is true, place a clean jam jar filled with clean water and screw the lid on. Leave it in direct daylight and over a period of time it will start to go green with algae. The water companies will say the algae is in the air and not in their water supply. If the water came from a tap and the lid screwed - you can make your own mind up. Either way, in the quantities if freshly drawn tap water there isn't a problem.
If renamed as 'pollutants', the minerals and other particles in suspension actually give the water its taste. Pure H2O is revolting to drink. Whilst on the subject bottled water also contains minerals and pollutants. Many are very high in salt. On the labels the salts are often masked by using their chemical names.
If the water is being used for cooking or drinking obviously putting bleach in it is not a good idea. However, when cleaning work surfaces, door handles, around the loo and so on bleach, or products containing bleach are advertised as killing 99.9% of all known germs.
But what is a germ? Basically there is no such thing as a germ. The word became associated with cleaning back in Victorian times. Most of the population had some rudimetary knowledge of how plants grew from seeds. The seed had to 'germinate'. So the advertising people promoted their products such as soaps and various other chemicals as killing 'germs'. Things that made you ill and kept growing.
Now in the 21st Century we are more aware of bacteria and viruses (If you lived through COVID period especially). Bacteria does not grow from a seed. It doesn't germinate. It replicates. Viruses are completely different to bacteria, but they do not grow from seeds either.
Moulds, fungi, mildew, yeasts are common place all over the planet. Some are toxic to animals, whilst others are food - mushrooms for example. They do not grow from seeds either. They multiply / duplicate from spores.
The question again: Will bleach kill them all?
No. Bleach certaily does kill many living things depending upon the concentration. Bleach in toothpaste for example that can lighten various colours to make teeth look more white. Bleach used on hair to strip any colour are just two examples. The water companies also use chemicals to kill off bacteria before pumping potable (drinking) water around to the consumers.
Mould spores are floating on the air continually. So small, they are invisible to the naked eye. Pollen at certain times of the year also float in the air. In very small amounts they will cause a reaction to some people and not others. Hay fever for example is just one of many.
Those spores and pollens are depositing everywhere. If moisture is available they may be able to absorb it. Spores of moulds can remain alive for decades, even hundreds of years dormantly awaiting moisture. (Whether it is true or not I do not know, but have read that spores on grain found in ancient burial sites have grown after thousands of years of dormancy).
Back to the kitchen worktops. You want to clean them off. You use a spray containing bleach. The spray will kill most spores, if not all it comes into contact with. But just like any battle field unless 100% of the enemy are put out of action you haven't won.
Mould spores swell in contact with moisture. They split and produce microscopic hair like strands that go in search of more moisture. Unlike seeds, spores carry no luggage. They haven't got a packed lunch to feed on until they can find food.
Mould spores absorb moisture and where possible anything that has carbon in its make up. Most moulds feed on carbon atoms. Sugar contains carbon and lots of it. Paper, and any material made from tree pulp contains sugar - cellulose. Most tile grouts, mastics, and tile adhesives contain carbon atoms. Many, if not most plants are full of sugar which is full of carbon.
Glucose, fructose, cellulose all contain carbon. Most plants are made up of those sugars. Cotton and flax used to make clothes contain quantities of carbon.
Butyl mastics and Silicone mastics contain carbon. The gaskets used to seal glazing units into window frames contain carbon. Therefore, the food supply for mould is virtually everywhere. That means unless you can kill all of the spores that have arrived and continue to arrive you will not win the battle.
[Say there is a colony of just 100 spores still alive behind the kitchen taps. There is steam in the air from cooking which condenses on the tap pipes when cold water goes through. Mould can replicate in about 20 minutes in the right conditions. 100 spore becomes 200 in 20 minutes. They become 400 after 40 minutes and 800 in the hour.
After only 8hrs the number of spore in the colony have become 1,677,721,600. Some moulds are visible if they have colour: typically white, green, yellow, black. In those numbers they will probably be emitting a musty smell as well.
Moulds are not bacteria. They are very different. The only thing they have in common is how quickly they can multiply though. Bacteria are extremely small and require high magnification to identify them. Mould spores tend to be much bigger].
Bleach works whilst it is in liquid form. As dry chemicals it is virtually inactive. Spraying the worksurfaces will for a while stop any spores from multiplying. They are still settling on the dry surface though. The spores you haven't killed can still multiply if they have food and moisture.
Reduce the relative humidity and it should reduce the water supply for the mould.

Reducing condensation will effectively reduce the moisture mould requires. Bleach, although will kill mould spores on contact isn't really practical to keep soaking your home.
Bleach does not remove calcite, it will take the colour out so it appears to have been removed. Acid is required. Very dilute hydrochloric acid in sprays such as Vyakal will remove thin deposites of calcite. Some loo cleaners that state they will dissolve limescale will, if used regularly.
Conclusions:
- Opening a window doesn't actually reduce much condensation. It all depends upon what the relative humidity outside and temperature actually is.
- Moulds multiply by replication and need moisture. They do not have multi-cellular seeds requiring germination.
- There are no such things as germs. They are in the same box as fairies, hobgoblins, bogeymen and so on.
- Bleach will not remove calcite (limescale). Dilute Hydrochloric acid is required. Vinegar will dissolve calcite, but will take a lot longer to do so.
- Relative humidity should be about 40 to 45%RH ideally. The occasional peak to 80%RH may occur directly after bathing, but should fall quickly when an extractor is pulling the humid air out of the home. [today - 15th August 2025; London air has a Relative Humidity recorded as 79.9%RH]
- Humid air is less dense than dry air at the same temperature and pressure. However, energy levels within the molecules also has a factor. Humidity in air diffuses, therefore it also fills spaces right down to floor level. It is often mis-diagnosed as 'rising damp'.
- To reduce condensation the water vapour must be removed from the home.
